Advances in COPD: a glimpse of the future
Dr Steve Holmes
Dr Steve Holmes
MMedSci, MBChB, FRCGP, DRCOG
Education lead, Primary Care Respiratory Society UK, Clinical Respiratory Lead Somerset CCG, GP Partner The Park Medical Practice, Shepton Mallet, Somerset
Dr Campbell Murdoch
MBChB, MRCGP
Somerset CCG Person Centred Care Lead, GP Partner, Wells Health Centre, Wells, Somerset
COPD is one of the most common long term conditions seen in primary care – but are we really addressing our patients’ needs with our current models of care? Evolving understanding of COPD phenotypes provides a glimpse into the future models of care being developed to improve quality of life for our patients
Chronic Obstructive Pulmonary Disease (COPD) is a common long term condition. It is usually associated with a history of cigarette smoking – but not all smokers develop the condition, and around 10-20% of people with COPD develop the condition without smoking. It is characterised by symptoms of breathlessness on exertion, cough at times, and flare ups (exacerbations) of increased breathlessness, cough, and often a change in sputum colour.
It is estimated that there are around three million people with COPD in the UK, but only around 1.2 million are currently diagnosed. We urgently need to improve COPD care in the UK: our premature mortality rate is almost twice the European (EU-15) average, and COPD is currently the second most common cause of emergency admission to hospital and one of the most costly diseases in acute hospital care in England.1
This article will examine factors that demonstrate quality of diagnosis in COPD, and areas we should consider in a person who has COPD and is more breathless (both acutely and chronically) than we would, perhaps expect – a common scenario in clinical practice. We will also cover the rationale for identifying COPD and key ways to help identify other common co-morbidities associated with it. We will then look at easily identified phenotypes that can be managed in primary care, prior to covering the wider, (and arguably) more important issues that our patients face. We will also cover a range of clinical phenotypes (traits/characteristics) that we can identify in people with COPD.
DIAGNOSIS – THE CHEFS CHECKLIST
A diagnosis of COPD is the start of interventions to optimise lung function and overall health. Expensive medications, use of steroid courses (with their potential for long term side effects), immunisations and rehabilitation programmes will be under consideration. If we get the diagnosis right we can use resources effectively. If we get it wrong we will be wasting resources, initiating treatments that have potential long term side effects and ignoring the underlying cause of the symptoms.
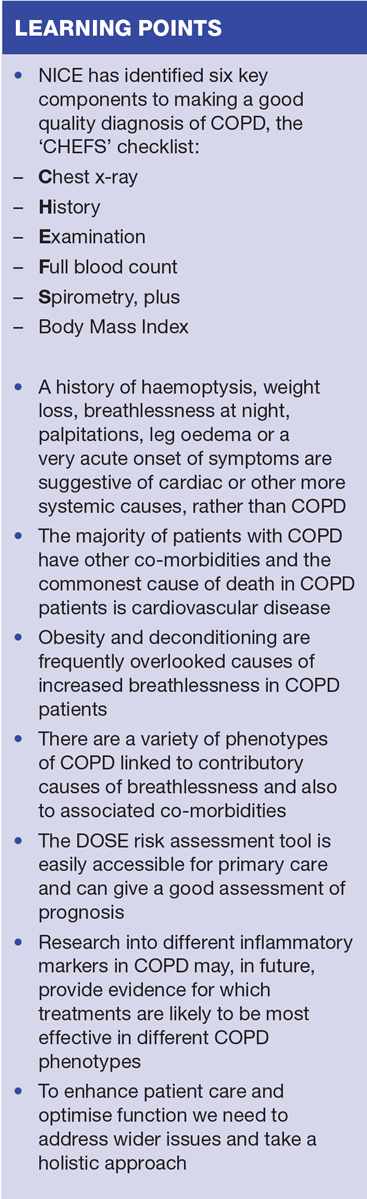
NICE has identified six key components to making a good quality diagnosis of COPD, the CHEFS checklist (Box 1).2 The sixth item (body mass index, [BMI]), which is not used in the acronym, would not affect the diagnosis but is an appropriate part of management and monitoring.
Chest X-ray
At the time of diagnosis it is sensible for the patient to have had a chest X-ray within six months. This should be repeated if there is a recent change or worsening of symptoms. Chest X-ray will, at times, reveal other diagnoses. Common differential diagnoses are:
- Carcinoma of the lung
- Heart failure
- Rarer lung disease, such as interstitial lung disease
- Other fibrotic conditions
- Bronchiectasis
- Pneumonia
There are, however, two important, practical tips for primary care regarding chest X-rays:
1. When symptoms or examination are suggestive of bronchiectasis, some form of interstitial lung disease or carcinoma of the lung, the chest X-ray may be entirely normal. The patient should be referred for more specialist investigation
2. The diagnosis of COPD is not made on the basis of a chest X-ray report that suggests ‘compatible with COPD’ or ‘hyperexpanded chest suggesting COPD’. The patient should be evaluated and have confirmatory spirometry.
History
There is usually a history of smoking exposure for a considerable period of time. If this is not the case we need to be very careful in establishing the diagnosis. Biomass fuels are a well-recognised risk factor in some countries; cooking on oil, wood or coal burning stoves in enclosed spaces, where smoke swirls around the cooking area, is a cause of COPD in non-smokers. It is also seen in people who have had untreated or undertreated asthma.3,4
The history is commonly of an insidious onset of exertional breathless over several years. It has recently been described as ‘a story with no beginning and no end’ as the symptoms of breathlessness are very gradual in onset.5 Chronic, slowly progressive symptoms are, however, commonly interspersed with exacerbations of breathlessness in association with cough and increased sputum production – sometimes treated as wheezy bronchitis.
You should be wary of a history of haemoptysis (carcinoma of the lung until proven otherwise), weight loss, breathlessness at night, palpitations, leg swelling or a very acute onset of symptoms, as these can be suggestive of cardiac or other more systemic causes.
Examination
Examination is a vital component in diagnosis and it is important to examine our patients carefully and thoroughly. This should include general appearance and the hands; specifically looking for anaemia, finger clubbing, tremor, nicotine stains and a variety of other cues, which help us appraise other potential causes. We should record pulse, blood pressure and examination of the heart and lower limbs, as well as the lungs; looking for cardiac causes for breathlessness, (e.g.heart failure, hypertension, valvular heart disease, atrial fibrillation), and anaemia.
Full blood count
NICE guidance also suggests we should have performed a recent full blood count, primarily to exclude chronic anaemia as a cause of breathlessness.2 Increasingly though, researchers are interested in eosinophil counts as we look for biomarkers that indicate a likely response to inhaled corticosteroids in people with asthma and COPD.6
Spirometry
Finally, COPD is confirmed (rather than diagnosed) with supportive, high quality spirometry.
There are two components to high quality spirometry:7
1. A reliable, reproducible test using validated and calibrated equipment
2. Ensuring that the interpretation assesses the quality of the spirometry, the parameters and what they could mean, and places this in context of the whole clinical picture to make a good, reliable diagnosis
THE BREATHLESS PATIENT WITH COPD
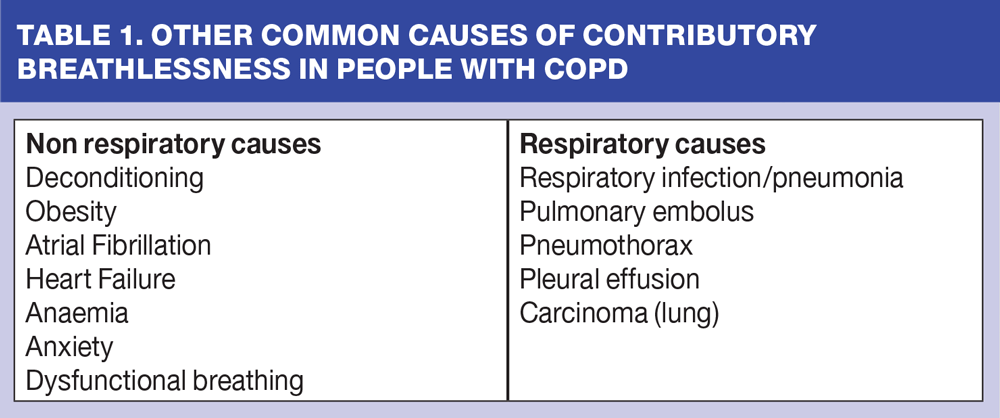
When we see a patient with COPD for review we need to be wary. We should not always attribute their breathlessness solely to COPD. This demands expert clinical thinking and a high index of suspicion. Breathlessness can be both acute and chronic but can often overlap into a mix of acute and chronic. A list of some of the more common causes are identified in Table 1.
A common, often overlooked, cause is obesity. Consider how breathless we feel carrying an extra 20 kg of weight for any distance, or when walking upstairs. This is the sort of weight that a person carries in a suitcase when going on holiday. It can be hard to imagine carrying this around all the time. Many of our patients carry significant amounts of excess weight and this will make it much harder for them to undertake tasks without getting breathless.
Similarly, most of us are aware of deconditioning but do not commonly apply this to our patients with COPD. An athlete or runner who does not exercise for a period of time will need quite a period to get their conditioning (fitness) back. The same applies to our patients with COPD. They may not be able to exercise for a variety of reasons (arthritis, fear of breathlessness, flare ups, lack of motivation), but dramatic improvements in breathlessness and activity can be achieved if they are supported to exercise more.8
Other medical causes of breathlessness need to be considered if our patients experience any step change in breathlessness.
MULTIMORBIDITY AGENDA
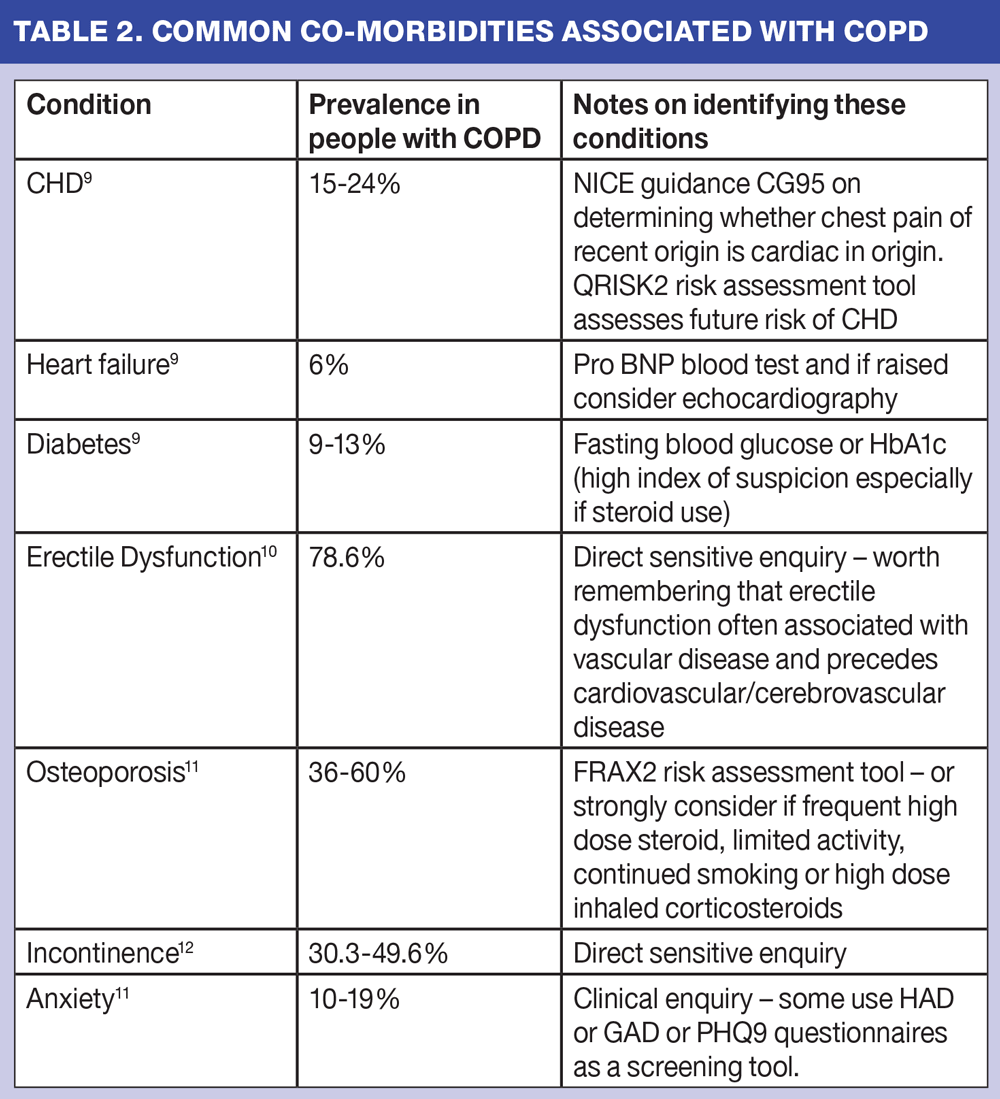
When thinking about phenotypes in COPD we also need to remember that there will be those who have coronary heart disease, heart failure, diabetes and potentially a mix of many of these. Indeed, recent research has suggested that only 14% of patients with COPD suffer only from COPD. The majority have other co-morbidities that can influence their symptoms and therapeutic options. The common co-morbidities are listed in table 2.
We need to recognise that the commonest cause of death for people with COPD is cardiac disease and should look to identify and, where possible, reduce this risk with pharmacological interventions.
MANAGEMENT STRATEGIES FOR DIFFERENT COPD PHENOTYPES
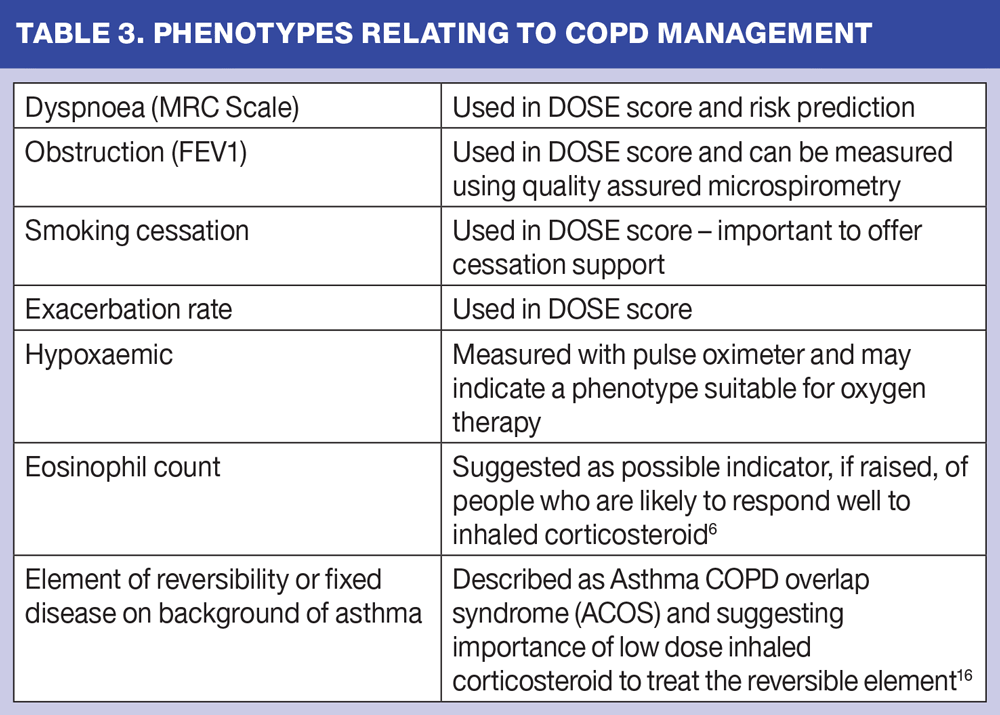
There are a variety of phenotypes of COPD linked to contributory causes of breathlessness and also to associated co-morbidities. When considering the disease and its evaluation, it is possible to identify a wide variety of factors and phenotypical traits that can help us manage our patients more effectively. (Table 3)
There is a well validated risk assessment tool, which, though not at present widely used in primary care in the UK, is easily accessed and predicts increased risk of morbidity and hospital admission.13,14 It assesses risk in a similar way to the most widely used secondary risk assessment tool, BODE.15 BODE predicts risk by evaluating:
- Body Mass Index – if the patient has lost a lot of weight their risk of death and admission increases
- Obstruction – the lower the FEV1 the greater the risk
- Dyspnoea – the Medical Research Council scale is commonly used and the more breathless a patient is, the worse the outcome
- Exercise capacity
Unfortunately the exercise test used in BODE is the 6 minute shuttle walk test. This is not particularly feasible in primary care, and also seems to be rarely reported, outside of research, in UK secondary care practice.
The primary care tool has the acronym DOSE. Dyspnoea and Obstruction – similar to the BODE index – but with Smoking status (smokers appear to do less well than those that have stopped) and Exacerbations (the more exacerbations the worse the outcomes). In clinical practice most of us can easily access DOSE and can gain a good idea of prognosis from this. Interestingly, there is no evidence that all the other parameters we measure with full spirometry (after making the diagnosis), except for the FEV1, have prognostic significance.2 In clinical practice the importance of checking for hypoxaemia with a pulse oximeter is also recognised, with consideration of full oxygen assessment if the patient has a saturation level of 92% or less.
There is increasing interest in determining which patients with COPD will benefit from inhaled corticosteroids, in association with bronchodilators. A recent literature review,6 has found increasing evidence to suggest that those who have a raised blood eosinophil count have a better response to inhaled and oral corticosteroids, compared with those with normal levels. This is still being debated, but increasingly it is suggested that we will, in future, be looking at other inflammatory parameters to determine our treatment.
In 2014, a collaborative group produced guidance on suggested management of the asthma/COPD phenotype, which they termed asthma COPD overlap syndrome (ACOS)16 – a condition that has probably been recognised as a conundrum in the real world of clinical practice for many years. The key management strategy here has been to suggest that if there is an element of reversibility it is important to consider inhaled corticosteroid (usually at lower doses) in addition to bronchodilation. (Table 3)
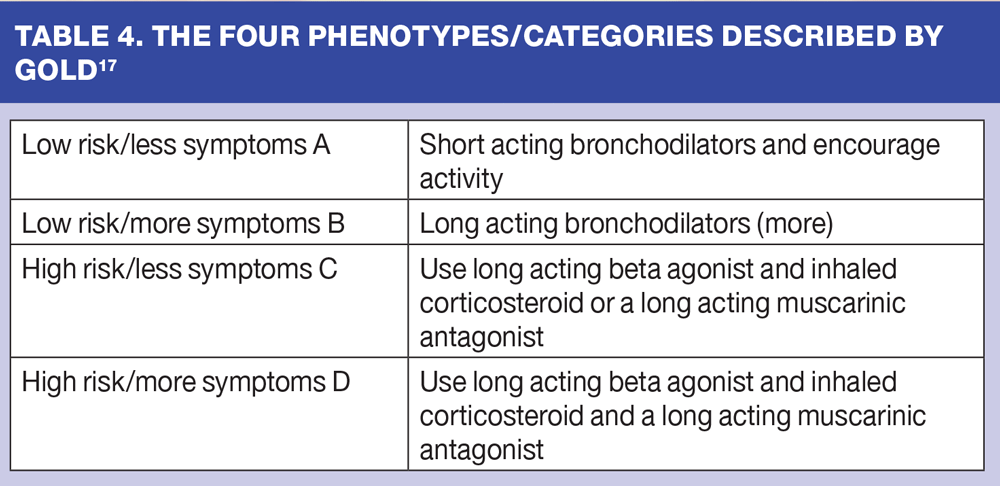
The current NICE clinical guideline is a partial update in 2010 of the original full review of 2004.2 People are now questioning whether we should be using this as an evidence base when so much more has been published since 2004. Indeed NICE is about to undertake a review of the need for further guidance in the near future. A considerable body of the respiratory world in the UK has been looking with interest at the GOLD guidance,17 which, while it does not fulfil the criteria for a high quality guideline in that the representative body of clinicians and patients is not really achieved and there is no systematic literature review process, does have good validity and links closely to the validated DOSE score in looking for how to treat patients with COPD.
GOLD highlights four phenotypes using a description of 0-1 exacerbations or mild-to-moderate obstruction (FEV1) as low risk, and 2 or more exacerbations or severe-to-very severe obstruction (FEV1) as high risk. (Table 4)
MANAGING THE WHOLE PERSON
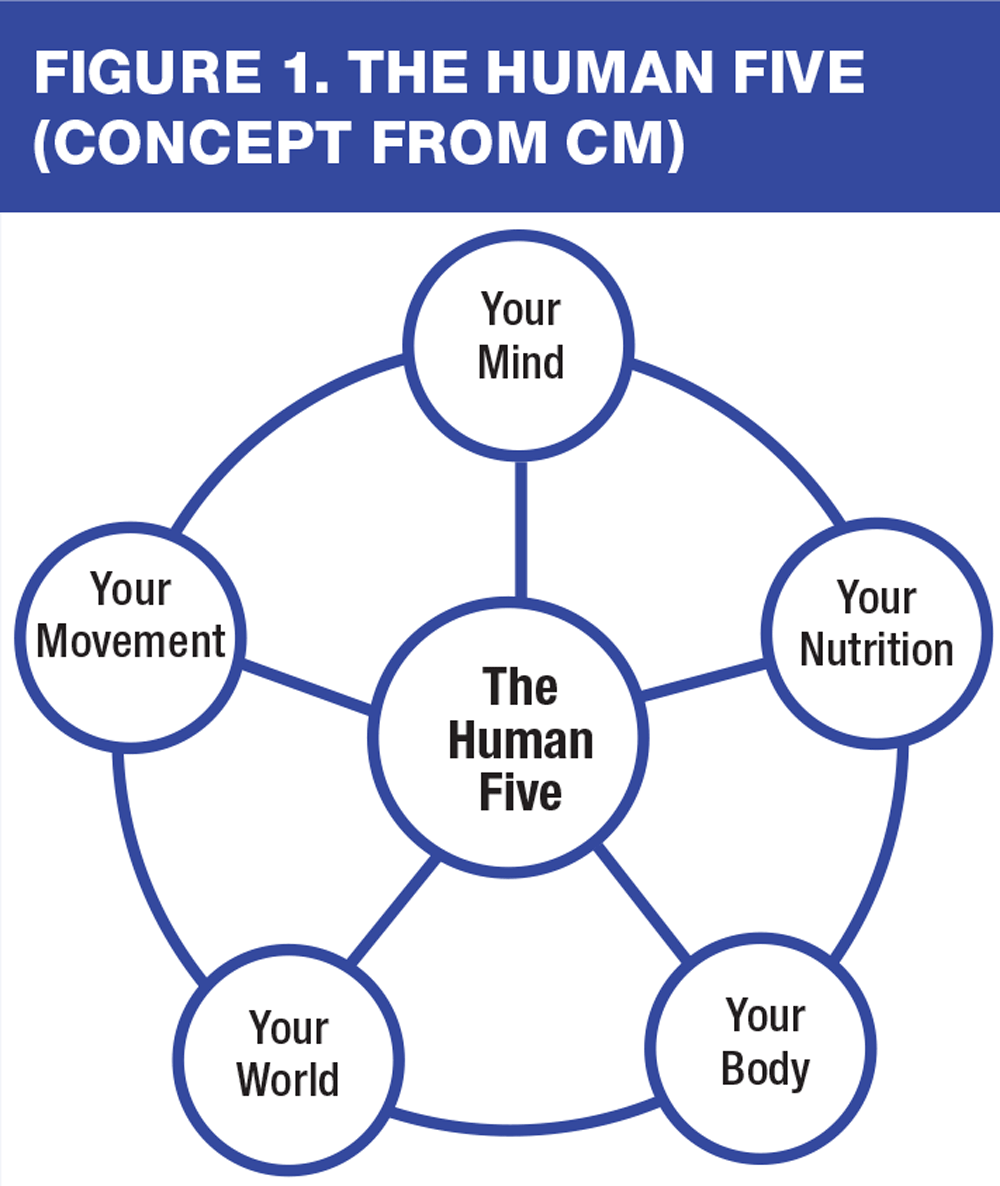
Many of us, at some stage in our career, have come across the importance of addressing the physical, social and psychological aspects of health and illness amongst our patients,18 a concept which dates back to the 1970s.19 To manage our patients more holistically a model, originally developed by one of the authors of this article (CM), can be adopted to enhance patient care and facilitate discussions with patients and carers in order to optimise function. Our patients are not alone in their society and several key factors influence their ability to function as well as possible. This model helps the clinician to address the factors that are key to human life and it can be seen from Figure 1 (page 37) that these are all inter-related.
Your body
This helps the clinician and patient consider the implications not only of their COPD but also co-morbidities, and the impact these have on their ability to function.9 It allows us to discuss the illness and treatment options and use medical evidence for interventions likely to help. Indeed most of this article has concentrated on this element of care to date.
Your mind
Not only are their issues linked to anxiety and depression but the way our mind works – our feelings, beliefs, values, knowledge and education – impacts on how we react to other areas.20 The stigma of needing oxygen or a wheelchair in a public place can have a major impact on patients and their carers, as will becoming very breathless when walking with friends and colleagues who do not know you very well.
Your movement
There is a great deal of evidence that conditioning our bodies allows us to get much more out of them – making us less breathless, able to undertake more activity (gardening, shopping or getting around the house) as well as producing natural endorphins which enhance mood. Our patients should be encouraged to exercise, and the supportive environment of pulmonary rehabilitation can be especially helpful if there are concerns.2,8,21
Your nutrition
Eating healthy food not only improves many parameters of health (weight, cholesterol, blood pressure),22,23 it can also help to provide a person with COPD the energy they need to exercise more easily. An increasing number of supplements are being developed for people with severe COPD to provide the necessary calories and protein to counteract the additional energy required to breathe and maintain muscle bulk.24
Your world
A final area to consider is one that is incredibly important to our patients. This encompasses their friends, partners, carers and social relationships, their home conditions, environment, transport options and finances. It would also include smoking cessation and alcohol – vital to optimising care.
CONCLUSION
A person with COPD needs a personalised approach to their care if we are to make a difference. First of all this demands a robust, quality diagnosis. They then require rigorous assessment and support that encompasses their symptoms and other potential co-morbidities as well as their particular COPD phenotype. An understanding of their phenotype links to the actions we should take and therapies we should use.
As we move into the future our patients will expect us to support them as people in a much more global sense. COPD is a challenge at the moment and high quality care will continue to challenge us into the future. Personalised care, our medical skills and knowledge, in association with our holistic and other skills, should enable us to enhance our patients’ quality of life.
REFERENCES
1. Department of Health. An outcomes strategy for chronic obstructive pulmonary disease (COPD) and asthma in England. London: Department of Health, 2011. https://www.gov.uk/government/publications/an-outcomes-strategy-for-people-with-chronic-obstructive-pulmonary-disease-copd-and-asthma-in-england
2. NICE CG101. Chronic obstructive pulmonary disease in over 16s: diagnosis and management, 2010. https://www.nice.org.uk/guidance/CG101
3. Tan WC, Sin DD, Bourbeau J, et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: results from the CanCOLD study. Thorax. 2015;70(9):822-9. http://thorax.bmj.com/content/70/9/822.long
4. Sexton P, Black P, Wu L, Sommerville F, Hamed M, Milne D, et al. Chronic obstructive pulmonary disease in non-smokers: a case-comparison study. Chronic Obsructive Pulmonary Disease. 2014;11(1):2-9.
5. Pinnock H, Kendall M, Murray SA, et al. Living and dying with severe chronic obstructive pulmonary disease: multi-perspective longitudinal qualitative study. BMJ. 2011;342:d142. http://www.bmj.com/content/342/bmj.d142
6. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Resp J. 2016;47(2):410-9.
7. Levy ML, Quanjer PH, Booker R, et al. Diagnostic Spirometry in Primary Care: Proposed standards for general practice compliant with American Thoracic Society and European Respiratory Society recommendations. Primary Care Respiratory Journal. 2009;18(3):130-47.
8. Beauchamp MK, Evans R, Janaudis-Ferreira T, et al. Systematic review of supervised exercise programs after pulmonary rehabilitation in individuals with COPD (Provisional abstract). Database of Abstracts of Reviews of Effects. 2013; (1):1124-33. http://onlinelibrary.wiley.com/o/cochrane/cldare/articles/DARE-12013065250/frame.html.
9. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 2012;380:37-43
10. Kahraman H, Sen B, Koksal N, et al. Erectile dysfunction and sex hormone changes in chronic obstructive pulmonary disease patients. Multidisciplinary Respiratory Medicine. 2013;8(1):66. http://mrmjournal.biomedcentral.com/articles/10.1186/2049-6958-8-66
11. van der Molen T. Co-morbidities of COPD in primary care: frequency, relation to COPD, and treatment consequences. Primary Care Respiratory Journal. 2010;19(4):326-34
12. Newman DK. In men and women with COPD the presence of urinary incontinence is associated with poorer quality of life. Evidence Based Nursing. 2014;17(1):22-3
13. Chavannes NH, Jones RCM, Postma DS, et al. Using COPD multidimensional indices in routine clinical practice: DOSE meets all criteria. Primary Care Respiratory Journal. 2012;21(3):245-6
14. Jones RC, Donaldson GC, Chavannes NH, et al. Derivation and Validation of a Composite Index of Severity in Chronic Obstructive Pulmonary Disease: The DOSE Index. American Journal of Respiratory and Critical Care Medicine. 2009;180(12):1189-95
15. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350(10):1005-12
16. GINA, GOLD. Diagnosis of Diseases of Chronic Airflow Limitation: Asthma, COPD and Asthma – COPD Overlap Syndrome (ACOS), 2014. http://www.goldcopd.org/uploads/users/files/GOLD_ACOS_2015.pdf
17. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of COPD. 2016. http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html
18. Cahill P, Papageorgiou A. Triadic communication in the primary care paediatric consultation: a review of the literature. Br J Gen Pract. 2007;57(544):904-11
19. Working Party of the Royal College of General Practitioners. The Triaxial Model of the Consultation. RCGP, London. 1972
20. Dowson CA, Town GI, Frampton C, et al. Psychopathology and illness beliefs influence COPD self-management. J Psychosom Res 2004;56(3):333-40
21. Troosters T, Casaburi R, Gosselink R, et al. Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease. Am J Resp Crit Care Med 2005;172(1):19-38
22. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368(14):1279-90
23. Tabak C, Smit H, Heederik D, Ocke M, Kromhout D. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clinical & Experimental Allergy. 2001;31(5):747-55.
24. Planas M, Álvarez J, García-Peris PA, de la Cuerda C, de Lucas P, Castellà M, et al. Nutritional support and quality of life in stable chronic obstructive pulmonary disease (COPD) patients. Clinical Nutrition. 2005;24(3):433-41.
Related articles
View all Articles