Prescribing in renal impairment
Beverley Bostock-Cox
Beverley Bostock-Cox
RGN MSc MA QN
Nurse Practitioner Mann Cottage Surgery, Moreton in Marsh
Education Lead, Education for Health, Warwick
It is not just those nurses who sign their own prescriptions who have prescribing responsibility: any nurse who advises on medication, in any way, needs to be aware of their legal and ethical responsibilities and will need to consider the impact of a patient’s comorbidities on prescribing practice, even if they do not sign the prescription themselves
Nurses who prescribe take on an important but satisfying responsibility. In 2006, the Nursing and Midwifery Council published its Standards of Proficiency for Nurse and Midwife Prescribers.1 This detailed the competencies expected of nurse prescribers. This document was replaced at the end of January 2019 with the updated publication of the Royal Pharmaceutical Society’s Competency Framework for All Prescribers.2 Nurses who prescribe must be able to treat the people they are prescribing for with care and consideration, ensuring that safe and effective practice is maintained at all times. In many cases, consideration should be given to comorbidities that may impact on prescribing practice and one example of this is when patients have renal impairment (RI).Although nurse prescribers are by definition those who have completed a prescribing qualification, many general practice nurses will prescribe in loco doctoris– in other words, will recommend medicine changes (or continuation) based either on a review of a person with a long term condition, or in an acute setting such as an acute (so-called minor) illness clinic. Any nurse who advises in any way on medication, either prescribed or over the counter (OTC) should be aware of their legal and ethical responsibilities, even if they do not sign the prescription themselves.
THE ROLE OF THE KIDNEYS IN PHARMACOKINETICS
The term pharmacokinetics relates to the movement of drugs; how the body deals with drugs that have entered the system. Pharmacokinetics consists of several stages, including:
- Absorption
- Distribution
- Metabolism, and
- Excretion.3
There are many factors that can influence these stages, including the route of administration and the drug dose. The primary organ of drug metabolism is the liver, whereas most excretion takes place in the kidneys, so any disease or impairment of the function of either of these organs will affect drug levels in the body. The nephrons, glomeruli and tubules all play an important role in the excretion and reabsorption of drugs via the kidneys. All drugs have different clearance rates, depending on glomerular filtration, tubular secretion and tubular reabsorption.
Tests of renal function
When prescribing, consideration should be paid to the glomerular filtration rate (GFR). Exact and direct measurement of GFR is complicated, however, and laboratories will therefore estimate the GFR (eGFR) based on a range of measurements, such as serum creatinine level, age, sex and race.4
The eGFR is considered to be normal if it is over 60ml/min/1.73m2. If the eGFR is found to be less than 60ml/min a repeat should be carried out within 1-2 weeks in case this is the first sign of impending acute kidney injury (AKI). If there is no evidence of accelerated loss of eGFR, the test should be repeated after 3 months and persistently abnormal eGFR (all three tests are less than 60ml/min over a 90-day period or longer) a diagnosis of chronic kidney disease (CKD) can be made.5 In patients with a very high or very low muscle mass, however, eGFR should be interpreted with caution. GFR will be overestimated in people with reduced muscle mass, whereas an increased muscle mass will lead to underestimation of the GFR.6
An alternative way of assessing glomerular function is via creatinine clearance (CrCl). This can be calculated using the Cockcroft and Gault formula. Although the values used in eGFR and CrCl measurements appear very similar, they are not equivalent. In cases of abnormally low or high BMI (<18kg/m2 or >40kg/m2) CrCl is the preferred measurement to assess renal function.
Kidney function can also be assessed with the albumin-to-creatinine ratio (ACR), calculated by dividing the amount of urine albumin by the amount of urine creatinine. An ACR below 3mg/mmol in both men and women is considered normal.
DRUG EFFICACY AND TOXICITY IN RI
When prescribing in renal disease it is important to remember that if drug excretion is impaired there is an increased risk of drug accumulation, leading to toxicity. In addition, the risk of drug side effects can be increased in people with a reduced eGFR.
To illustrate this consider the use of some of the drugs used for type 2 diabetes (T2D). (Box 1)
THE INFLUENCE OF RI ON DRUG CHOICES
There are some simple points to remember when thinking about prescribing medication in people with RI:
- Only prescribe medication when there is a definite indication and where the benefits of treatment clearly outweigh any risks
- Once the decision to prescribe has been made, choose a drug that has the lowest risk of nephrotoxicity
- Use the recommended dosage regimens, based on resources such as the British National Formulary https://bnf.nice.org.uk/ or the Electronic Medicines Compendium (EMC) https://www.medicines.org.uk/emc/
- Carry out regular reviews to ensure that the drug is having a positive impact and that there is no evidence of toxicity.
In some cases, however, the risk: benefit profile may take on a different perspective, e.g. in palliative care. Someone who is dying from heart failure may need to take high doses of potent diuretics to treat fluid overload. This needs to be done with the full knowledge and acceptance of both the clinician and the patient and/or their family that this will impact negatively on their renal function. Treating breathlessness and oedema, however, may be more important at the end of life than worrying about the kidneys. In cases such as these, good communication skills are essential, along with forward planning and proactive discussions earlier on in the disease process.
THE IMPACT OF RI ON DRUG DOSES
Dose recommendations are based on the severity of RI. The total daily maintenance dose of a drug can be reduced either by reducing the size of the individual doses or by increasing the interval between doses. A key concern will be reducing the risk of AKI.
When people with an eGFR of less than 60ml/min become acutely unwell, the risk of developing AKI is increased. This issue is reflected in the STOPP START guidance,12 which encourages clinicians to consider reducing or stopping drugs which are known to be toxic to the kidneys and/or are excreted via the kidneys. There is a commonly used acronym for remembering the drugs that should be stopped in people who may be at risk of AKI: Stop the DAMN drugs, where:
D = diuretics
A = ACE inhibitors and angiotensin receptor blockers (sartans)
M = metformin (but this would now also include the SGLT2 inhibitors)
N = non-steroidal anti-inflammatory drugs (NSAIDs).
These drugs are known to increase pressure on the kidneys in concurrent acute illness. Clinicians should therefore consider stopping them (or at the very least reducing the doses) until the individual has recovered, at which point, consideration should be given to restarting them. However, particular consideration should be given before restarting drugs such as NSAIDs. According to NICE, the use of NSAIDs in people with CKD can lead to further deterioration in renal function.5 Even acute, short term use of NSAIDs can have a deleterious effect. NICE therefore recommends that the effects of NSAID use on GFR should be monitored carefully, particularly in people who already suffer from renal impairment or who have other risks for CKD.5
FOLLOWING UP PEOPLE WITH RI
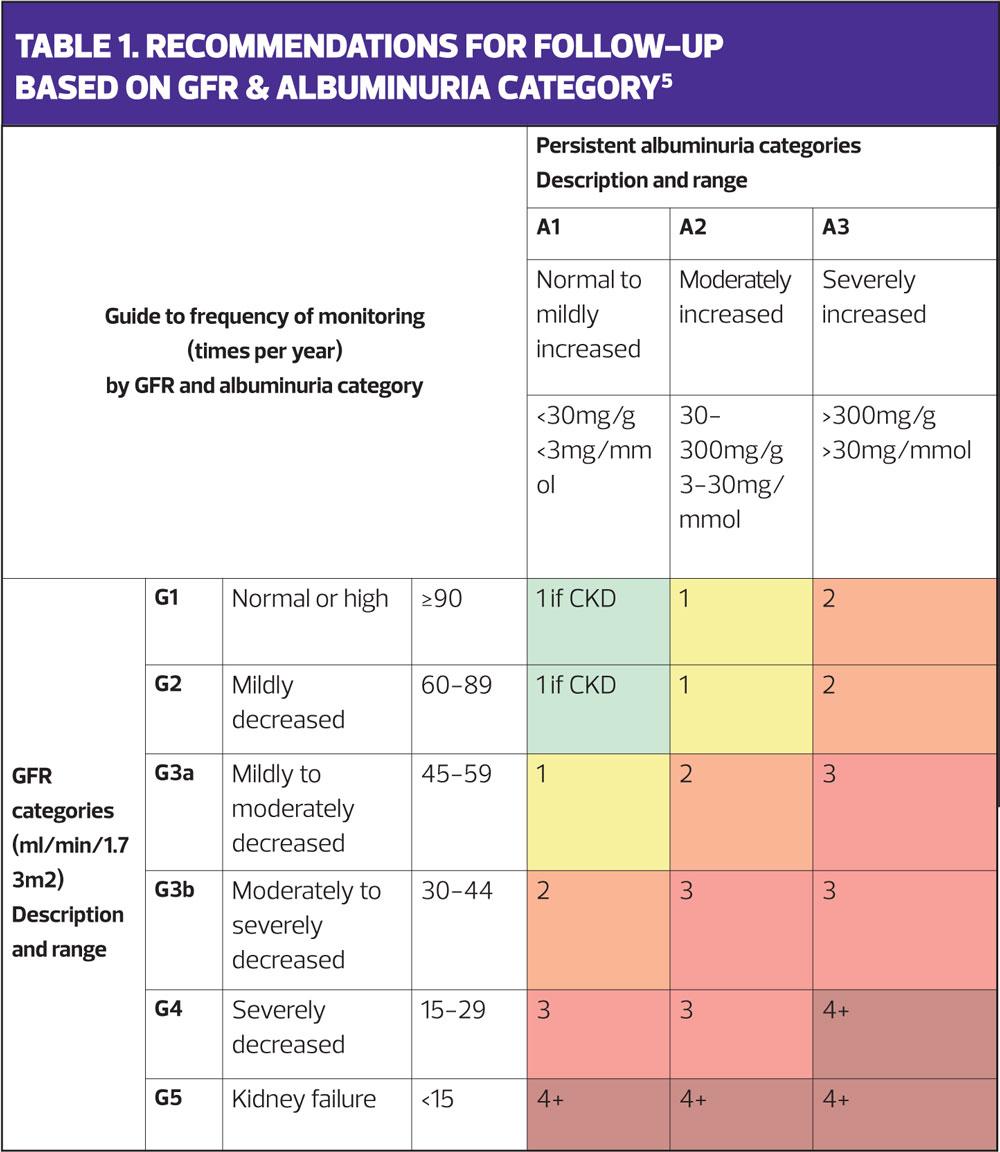
Table 1, from the NICE guideline,5 indicates the number of times per year that people with CKD should be monitored, although this can be tailored to meet the needs of each individual. Each review should prompt the clinician to consider the patient’s current medication and if, or how it might need to be adjusted if the renal function has altered. Reviews should also include lifestyle advice such as smoking cessation, weight management and encouragement to follow a healthy eating and activity programme which will improve holistic well-being as well as kidney health.5 As people with CKD are at high risk of cardiovascular disease, both lifestyle and pharmacological interventions should be discussed with a view to reducing these risk factors.5
CASE STUDIES
It would be impossible to give a full and conclusive list of the drugs that should be reviewed in people with RI because there are so many variables: age, comorbidities and polypharmacy, to name but a few. However, the examples here are cases that may be commonly seen in general practice and where a review of the suitability of the medication and/or doses should be carried out when RI has been diagnosed.
Case study 1. Daniel
Daniel, age 63, has angina and is treated with bisoprolol 10mg and atorvastatin 80mg. He also takes ramipril 10mg and aspirin 75mg. He takes ibuprofen 400mg three times a day, as needed, which he buys over the counter (OTC). His renal function has been declining over the years and his latest eGFR is 52ml/min/1.73m2. Last year at his annual review, it was 55ml/min. His ACR is 0.6 mg/mmol.
Before reading further look at Activity 1.
According to the NICE chart, (Table 1) Daniel is CKD category G3a A1 and he needs reviewing annually.
The EMC website makes the following recommendations about each of his drugs:
- Bisoprolol 10mg: dose adjustments should be based on CrCl, not eGFR so this should be assessed for accuracy. This can be done via the practice computer system or via an online calculator. The EMC recommends that dose adjustment should only be considered in people with a CrCl < 20 ml/min (severe RI) where the dose should be capped at 10 mg once daily (the maximum being 20mg). This dosage may be divided into halves to optimise the risk: benefit ratio. In essence then, Daniel can stay on his current dose of bisoprolol.
- Atorvastatin 80mg: no dose adjustment is required.
- Ramipril 10mg: once again, dose adjustments are based on CrCl. The EMC website advises that if creatinine clearance is between 30-60ml/min, the maximum daily dose should be 5mg. On that basis, Daniel’s CrCl should be calculated and the dose is likely to need adjusting down to 5mg.
- Aspirin 75mg: aspirin is contraindicated in severe RI so for now, Daniel’s prophylactic dose of 75mg is acceptable. However, he should be warned about taking any OTC drugs which contain aspirin such as pain killers or cold remedies.
- Ibuprofen 400mg: the EMC advises against long term use of NSAIDs because of a range of possible adverse drug reactions, including renal impairment. It states that taking NSAIDs may cause a dose dependent reduction in prostaglandin formation, which may then lead to acute renal failure. It advises that the patients most at risk of this adverse drug reaction are those who already have impaired renal function, but may also include anyone with cardiac impairment, liver dysfunction, those taking diuretics and the elderly. EMC recommends that in these cases the lowest effective dose should be taken for the shortest possible time and that renal function should be carefully monitored. Ideally, a discussion should ensue to determine why Daniel takes these drugs and how often. The potential risks should be explored with him and safer options investigated, such as those that can be found at Versus Arthritis. If he makes an autonomous decision to continue on his NSAIDs he may need his kidney function reviewing more frequently than annually.
Case study 2. Lenny
Lenny, aged 78, has been diagnosed with hypertension and takes a calcium channel blocker, amlodipine 5mg, for this. He also has chronic obstructive pulmonary disease (COPD) and takes a long acting muscarinic antagonist (LAMA), tiotropium 5mcg, once daily for this condition. His renal function is impaired but has been stable with an eGFR in the low 40s for the past 4 years. His latest reading is 42ml/min/1.73m2. His ACR is 2mg/mmol. He has come to see you for a COPD review but mentions he has a cold and has been taking an OTC cold remedy for this.
Before reading further look at Activity 2.
Lenny’s CKD category is G3b A1 and he needs to be reviewed twice a year. Regarding his drugs, the EMC advises the following:
- Amlodipine 5mg: amlodipine is metabolised into inactive metabolites and amlodipine doses are not affected by renal impairment. Therefore the normal dosage is recommended in patients with CKD.
- Tiotropium 5mcg: consideration needs to be given to each individual case. The EMC states that plasma concentrations of tiotropium have been noted to rise in patients with moderate to severe RI (CrCl ≤ 50 ml/min) so tiotropium bromide should be used only if the expected benefit outweighs the potential risk. Furthermore, they advise that there is no long-term experience in patients with severe RI. His use of tiotropium should be discussed with Lenny, although it is likely that he will benefit more from taking his LAMA than from stopping it. Other LAMAs do not have the same warning associated with them. For example, no dose adjustment is needed for umeclidinium or for aclidinium. Glycopyrronium has a similar warning to tiotropium, in that it says that in patients with severe or end stage RI the drug should be used only if the expected benefit outweighs any potential risk.
- Cold remedy: further investigation reveals that Lenny’s cold remedy, which is commonly bought OTC, contains paracetamol, pholcodine, promethazine hydrochloride, dextromethorphan hydrobromide and pseudoephedrine hydrochloride. The summary of product characteristics on the EMC website advises that any level of RI should elicit caution for use for this product and that it is contraindicated in severe RI.
Now look at Activity 3.
Case study 3. Cassie
Cassie, age 82, has a urinary tract infection (UTI) with polyuria, dysuria and dip stick confirmation of the presence of leucocytes, nitrite, blood and protein. This is her third UTI this year. She is known to have CKD and she also suffers from asthma, atrial fibrillation and bladder instability. She has been prescribed Fostair NextHaler 100mcg/6mcg 1 puff bd, rivaroxaban 20mg daily and oxybutynin 5mg bd. She has been diagnosed with moderate frailty and her mobility is limited. Cassie last had her bloods and urine test checked a year ago when her eGFR was 27ml/min/1.73m2 and her ACR was 32 mg/mmol.
Before reading further look at Activity 4.
Cassie was identified as being in category G4 A2 a year ago and, as such, should have had her renal function and her drugs reviewed at least 3 times since then. Her urinary symptoms deserve further investigations to determine the relationship with her CKD and unstable bladder and also to see if there were other reversible causes such as atrophic vaginitis or incomplete bladder emptying.13
The EMC advice about her current medication is as follows:
- Fostair (beclometasone/formoterol): Perhaps surprisingly, the EMC advises that there is no evidence about the use of either drug in RI and no dose adjustment is needed for age. The website does, however, state that as beclometasone dipropionate is metabolised very quickly, an increase in systemic exposure to this ingredient would not be anticipated in patients with RI. Once again, a consideration of the risk benefit profile should be carried out and, as asthma needs an inhaled corticosteroid (ICS) to control it, it is likely that the ratio of benefit to harm would be positive. However, it may be that Cassie only needs an ICS and the formoterol could be stopped.14
- Rivaroxaban: The EMC website states that the current data for patients with severe RI, which is described as a CrCl of 15 – 29 ml/min, shows that plasma concentrations are significantly increased. Rivaroxaban is not recommended in patients with CrCl < 15 ml/min. In patients with moderate RI (CrCl 30 - 49 ml/min) or severe RI (CrCl 15-29 ml/min) the recommended dose would be 15 mg once daily. Cassie needs her renal function reassessing as a matter of urgency, so that her dose can be adjusted accordingly and a holistic assessment of her well-being carried out.
- Oxybutynin: The EMC warns that oxybutynin should be used with caution in the frail elderly, or those with RI. A review of the management of Cassie’s bladder problems should be carried out, based on NICE guidance and recommendations. These can be found at: https://pathways.nice.org.uk/pathways/urinary-incontinence-in-women/managing-overactive-bladder-in-women
For advice on treating UTIs, including in adults, children, pregnant women and people with RI, the recently published NICE guidance on recurrent UTI is worth reading.15 These guidelines recommend using nitrofurantoin 100mg MR bd for 3 days as a first line option if the eGFR is 45ml/min or more. This is not the case for Cassie as her eGFR is only 27ml/min. Of note, the NICE guidelines recommend that this drug and dose may still be used with caution if the eGFR is between 30-44ml/ minute to treat uncomplicated lower UTI if the potential benefit is deemed to outweigh the risk.
Now look at Activity 5.
SUMMARY
The kidneys are the key organ for drug excretion and renal impairment can have a significant effect on drug levels, efficacy and toxicity. Drug choices and doses will be affected by the presence and severity of RI and people on medication should be assessed regularly to determine whether they are renally impaired and if so, how their medication should be amended as a result. All nurses should be aware of these issues and should be able to act within their code of conduct to individualise care, practise effectively and preserve patient safety.
The over-the-counter cold remedy taken by Lenny (Case 2) is Day and Night Nurse®
REFERENCES
1. Nursing and Midwifery Council. Standards of proficiency for nurse and midwife prescribers, 2006
2. Royal Pharmaceutical Society. Competency Framework for All Prescribers, 2019 https://www.rpharms.com/resources/frameworks/prescribers-competency-framework
3. Ritter JM, Flower R, Henderson G, et al. Rang & Dale's Pharmacology. 9th edition 2019 www.elsevier.com
4. The Renal Association. About eGFR. 2018 https://renal.org/information-resources/the-uk-eckd-guide/about-egfr/
5. NICE CG182. Chronic kidney disease in adults: assessment and management, 2014 (updated 2015) https://www.nice.org.uk/guidance/cg182
6. Dowling TC, Matzke JR, Murphy JE, et al. Estimated GFR vs Creatinine Clearance for Drug Dosing. Am J Kidney Dis 2009; 54(5): 984 - 985
7. Krepinsky J, Ingram AJ, Clase CM. Prolonged sulfonylurea-induced hypoglycemia in diabetic patients with end-stage renal disease. Am J Kidney Dis 2000; 35(3): 500-505
8. Russo E, Penno G, Del Prato S. Managing diabetic patients with moderate or severe renal impairment using DPP-4 inhibitors: focus on vildagliptin. Diabetes Metab Syndr Obes 2013;6:161-70. doi:10.2147/DMSO.S28951
9. Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators. N Engl J Med 2015; 373(22): 2117-28.
10. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644-657
11. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. Am Heart Jl 2018; 200:83-89
12. NHS England. Toolkit for general practice in supporting older people living with frailty, 2016
13. NICE CG171. Urinary incontinence in women: management, 2013 (updated 2015) https://www.nice.org.uk/guidance/cg171
14. British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. 2016 https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/
15. NICE CG112. Urinary tract infection (recurrent): antimicrobial prescribing, 2018 https://www.nice.org.uk/guidance/ng112