Primary Breast Cancer: what do practice nurses need to know?
Tessa Watts
Tessa Watts
BA (Hons), MSc, RN, PGCE
Senior Lecturer
College of Human and Health Sciences
Swansea University
New and emerging primary breast cancer treatment regimens, together with increased survival present fresh challenges for health professionals. To provide proactive, health promoting care and support, practice nurses need to be aware of treatments, and their immediate and long term effects. This article offers an overview of contemporary approaches to management.
Worldwide, breast cancer is the commonest form of invasive female cancer.1 Across Europe, since the late twentieth century, its incidence has continued to increase. Today it is the most common cancer among women in the UK, accounting for just under a third of new female cancer cases. Breast cancer in men is rare, accounting for less than 1% of male cancers.2
It is estimated that one in eight women will be diagnosed with breast cancer, with incidence increasing with age. Between 2007 and 2009, 45% of new cases were in women aged over 65. However, breast cancer is also the most frequently diagnosed cancer in women under 39 years of age. In recent years, the incidence of hormone receptor positive breast cancer among post-menopausal women has decreased.3-5 This has been linked with declining use of HRT.6 Although incidence is higher among women from affluent socio-economic groups, survival is lower in less affluent women.
Across the world, breast cancer is the main cause of death in women over 45,7 but, since peaking in the late 1980s, breast cancer mortality in Europe has decreased by 15%.8 Breast cancer survival rates have been increasing steadily, and 5-year survival is now estimated to be at least 80%.9 The decline in mortality is connected with early detection through increased breast awareness and screening, coupled with enhanced surgical and radiotherapy regimes, greater use of adjuvant anti-oestrogen therapies and more effective chemotherapy.10
RISK FACTORS
The causes of breast cancer are uncertain and primary prevention is not currently possible.11,12 Nonetheless, several genetic, reproductive, environmental and lifestyle risk factors have been identified.13,14 (Box 1) While there remains some ambiguity about how these cause cells to become cancerous, it would seem that there is a possibility for primary prevention, particularly in terms of lifestyle modification. Research is ongoing.
EARLY DETECTION
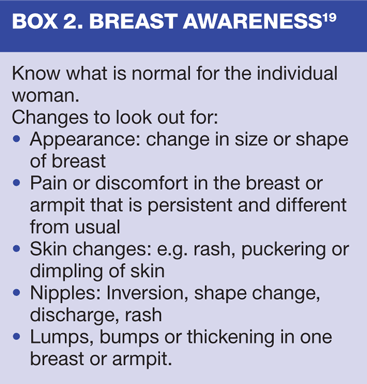
Early detection and swift treatment offer greater possibility for survival15 and enhanced quality of life. Thus, early detection through breast awareness or mammography assumes a central role.16 Women are now encouraged to be 'breast aware',17 (Box 2) This signals a move away from breast self-examination, which has been found to be of limited benefit and even harmful.18,19 Practice nurses are ideally placed to work alongside women, helping them to become breast aware through education, information and support.
In the UK, 3-yearly mammography screening for women over 50 was introduced in the 1980s. Initially, women were offered screening until they reached 64. In 2001 this was extended to 69. The decline in breast cancer mortality rates in the United Kingdom, particularly in women aged 50-64 may be attributable to screening-associated early detection.20 However, in recent months there has been controversy over the balance of benefits and harms of breast cancer screening, with some authors suggesting that it results in overdiagnosis and treatment, while others maintain that the risk of harms is overestimated. This uncertainty may deter women from accepting screening, so practice nurses have a job to do, to help women in making informed decisions by providing — as far as possible — evidence based, accurate information. Meanwhile, an independent review of the research evidence for mammography screening is underway.
DIAGNOSTICS
While most breast disease identified through screening is subsequently found to be benign,21 the diagnostic phase is a difficult time.22 Despite efforts to reduce the time between suspicion of cancer to confirmation of diagnosis and thus health status, this 'waiting game'23 is characterised by profound uncertainty and anxiety.24 Several studies have indicated that fear of cancer is a significant cause of psychological distress.25
Breast cancer diagnosis is made following combined 'triple assessment'. This assessment is normally undertaken in a single visit to a designated 'one stop' breast clinic. It encompasses:
- Clinical assessment by breast specialist
- Radiology — mammography or ultrasound
- Cytology — fine needle aspiration or core biopsy.26
Triple assessment provides rapid but accurate diagnosis, thus helping to prevent unnecessary surgery. Moreover, patients have access to specialist breast cancer nurse support.
TREATMENT OPTIONS
Awareness and understanding of breast cancer treatments can help you support patients, not only during treatment, but also when treatments are completed. For some people breast cancer and its treatment brings about a wide range of significant physical and psychosocial problems,27 including second cancers and more general issues relating to life after cancer. Some of these problems may not diminish over time and may even emerge long after treatment and routine specialist follow up has ended.28
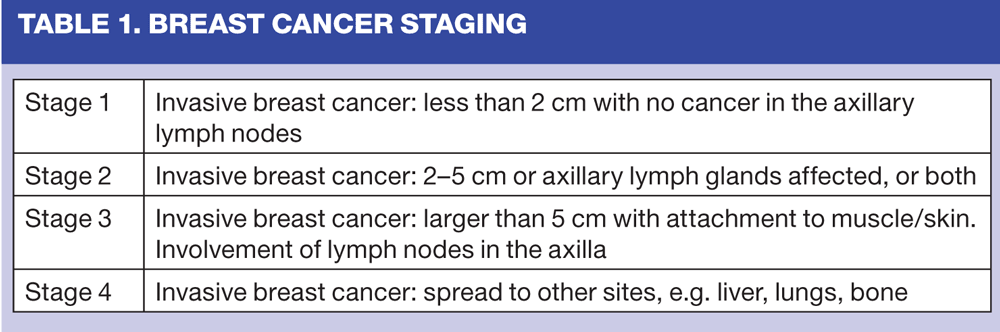
Breast cancer is a complex disease of many different types, each with its own specific biological features and prognostic indicators. Invasive cancers are classified on the basis of tumour type, histological grade, i.e. the nature of the cancerous cells, the size and spread of the tumour. (Table 1) Classification is extremely important in terms of treatment decision-making. It is also recommended that hormone receptor and human epidermal growth factor receptor 2 (HER2) status are assessed.29
In recent years considerable advances have been made in breast cancer treatments, notably in relation to new chemotherapy regimens, hormone and biological therapies. Thus primary breast cancer treatment may be a combination of local treatments, e.g. surgery and radiotherapy, systemic treatments, e.g. chemotherapy and endocrine therapy and, in some cases, newer, targeted treatments, e.g. trastuzumab (Herceptin). Treatments may take place over a long time period.30
Surgery
Treatment for primary, localised breast cancer usually involves surgery. Depending on tumour type, staging and size, surgery may be:
- Breast conserving surgery (wide local excision or lumpectomy)
- Mastectomy (simple, modified radical or radical).
With improved surgical techniques, surgery today is usually far less radical and invasive compared with the early Halstead mastectomy and the modified radical mastectomy (a less extensive mastectomy in which the pectoralis muscles are unharmed). For early, localised breast cancer, breast conserving surgery emerged during the 1980s and has become an accepted alternative to mastectomy, with similar outcomes in terms of survival.31 The increased use of breast conserving surgery has been associated with screening mammography; since the introduction of screening the average size of invasive tumours has decreased and the detection of small and/or non-invasive breast cancer (Ductal Carcinoma in Situ [DCIS]) has increased.32
Although surgery can remove disease in or around the breast, undetected deposits may remain, either locally — in residual breast tissue, scar area, chest wall, regional lymph nodes — or at distant sites. Thus surgery is usually followed by adjuvant radiotherapy, chemotherapy and/or hormone therapy. On occasion, adjuvant induction therapy might precede surgery.
In invasive breast cancer, axillary lymph node assessment is crucial to staging. It is a powerful prognostic indicator, and is also useful in adjuvant treatment decision making.33 Axillary lymph node dissection has been an integral part of breast cancer surgery for many years, particularly for those with node positive breast cancer. NICE advocates that axillary management is important to reduce the risk of local recurrence and enhance survival. However, axillary dissection or even clearance is not without possible adverse effects. The procedure is associated with pain, increased lifetime risk of ipsilateral upper limb lymphoedema, parasthesia and decreased range of movement. These problems have a significant impact on quality of life.34 Lymphoedema in particular can become a chronic, debilitating condition. (Box 3)
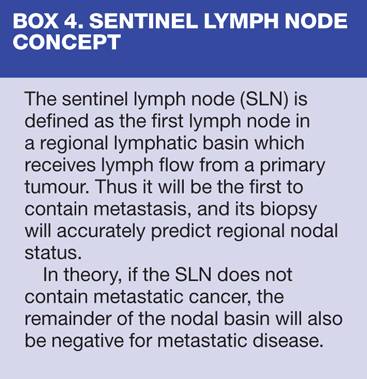
New techniques such as 4-node sampling and sentinel lymph node biopsy (Box 4) have emerged as alternatives. The latter is associated with a decreased incidence of lymphoedema.
Radiotherapy
External beam breast radiotherapy is an integral component of breast conserving treatment as the conserved breast is at risk of local recurrence, even when nodes are negative. This risk may be reduced by radiotherapy.35 Following breast conserving surgery or mastectomy in node-positive patients, radiotherapy is associated with reduced recurrence and increased survival.36
Although the aim of radiotherapy is to destroy tumour cells without damaging healthy tissue, the benefit is set against unpleasant, adverse physical effects, notably fatigue, acute skin irritation and pain.37 A recent Swedish study38 demonstrates that these problems can persist for some time following treatment completion and can impact on quality of life. As radiotherapy usually takes place during the working week over a number of weeks, and possibly at some distance from the patient's home, everyday life can be profoundly disrupted. Many women, not knowing quite what to expect or the potential side effects, are fearful of radiotherapy.39
Chemotherapy
Adjuvant chemotherapy is associated with reduced risk of relapse and death in women with early stage breast cancer,40 and is therefore recommended for women at significant risk of recurrence. The choice of chemotherapy agents is dependent on both tumour and patient characteristics. Combination treatment is usually offered. This is because chemotherapeutic drugs interfere with a cancer cell's ability to divide and grow and different drugs act at different phases of the cell cycle.
Regimes are individually tailored and administered according to protocol. Today the combination usually includes a drug from the anthracycline group, e.g. adriamycin or epirubicin, as such regimens have been shown to reduce recurrence and increase five year survival. In 2009, following clinical trials of drugs in the taxane group, NICE recommended docetaxel, administered concurrently with doxorubicin and cyclophosphamide in early lymph node-positive breast cancer.
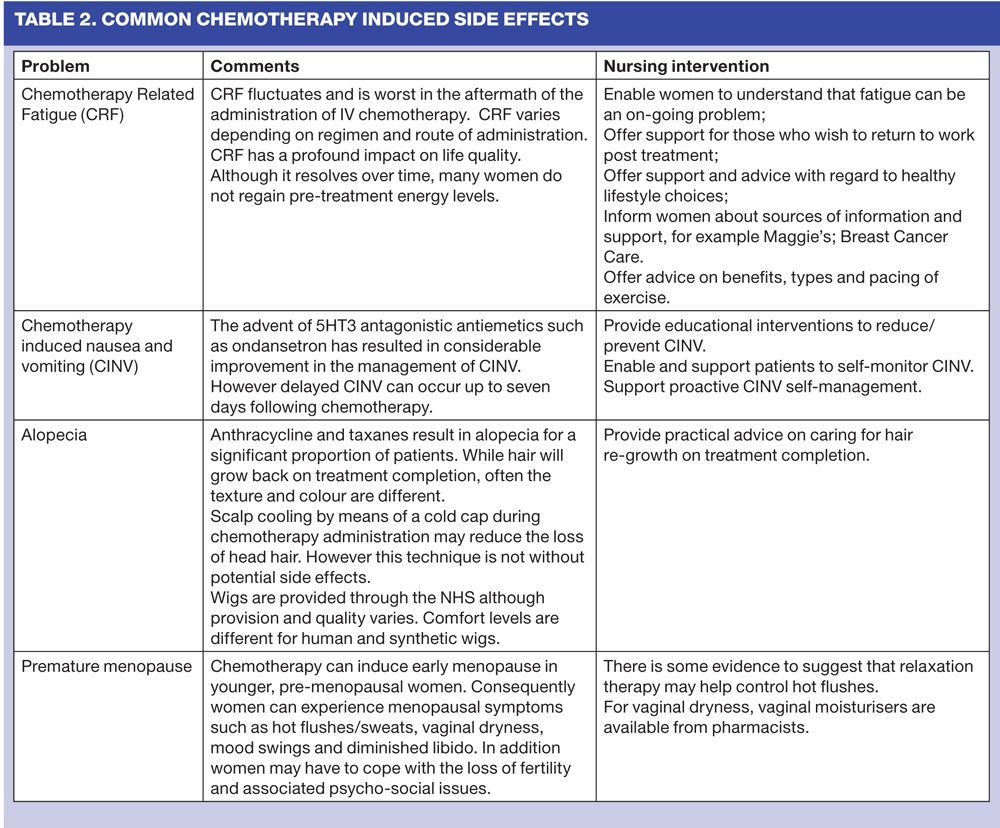
As chemotherapy may also affect normal cells, a range of unpleasant and debilitating, but predictable side-effects may be experienced. (Table 2) These can have a profound impact on the patient's quality of life. Side effects may arise during, between and immediately after treatments and are related to the type and dose of chemotherapeutic drug and its half-life, the route and schedule of administration and existing co-morbidities. However, some side effects e.g. fatigue, may continue long after treatment has ceased.
Today chemotherapy is increasingly administered near or even at the patient's own home. Consequently, chemotherapy induced side effects are likely to arise when the person is at home. To optimise outcomes it is therefore vital that primary care professionals, including practice nurses, acquire the necessary knowledge and understanding to effect safe, high quality care and appropriate, timely referral to specialist practitioners.
Hormone therapy
To reduce the risk of recurrence, some women are prescribed adjuvant endocrine therapy. Pre-menopausal women with hormone receptor-positive early breast cancer may be prescribed tamoxifen, a selective oestrogen receptor modulator. A recent meta-analysis41 showed that five years of adjuvant tamoxifen reduces the 15-year risk of breast cancer recurrence and death. However, this advantage applies only to women with oestrogen receptor-positive disease. Moreover, while the oestrogenic effect of tamoxifen confers some benefit to post-menopausal women, for example, reducing osteoporosis, its side effects, e.g. hot flushes, mood disturbance and fatigue, have been shown to have a negative impact on quality of life.42
In post-menopausal women with oestrogen receptor-positive early breast cancer, aromatase inhibitors, e.g. anastrozole (Arimidex), are now recommended. By inhibiting aromotase activity, these drugs decrease oestrogen production in peripheral tissues. Effectively, oestrogen-sensitive breast cancer cells are starved. Nonetheless, while aromatase inhibitors do not appear to cause the type of gynaecological effects associated with tamoxifen, musculoskeletal pain and osteoporosis may be experienced.
Biological (targeted) therapy
The HER2 gene is present in about 15—20% of early stage invasive breast cancers.43 HER2 is a receptor for the naturally occurring human epidermal growth factor. When this growth factor attaches itself to HER2 receptors on breast cancer cells, cell division and growth is stimulated. The result is more aggressive breast cancer, resistant to conventional hormone and chemotherapy treatments.
The advent of treatments specifically targeted at HER2 has substantially improved outcomes for HER2-positive patients. Trastuzumab in particular has been found to confer benefit for women with early and advanced stage HER2-positive breast cancer. Trials to define and optimise scheduling and duration of approved HER2 targeted treatments in early HER2 positive breast cancer patients are ongoing. In the meantime NICE recommends that, following cardiac function assessment, trastuzumab is given at 3-week intervals for a year or until disease recurrence (whichever is the shorter period), as an adjuvant treatment to women with HER2-positive early invasive breast cancer following surgery, chemotherapy, and radiotherapy, when applicable.
SUPPORTING SURVIVORS
Advances in early detection and treatments mean that many more people are surviving, and will live healthy, long lives following primary breast cancer treatments. Ironically, the treatments that enhance breast cancer survival can have adverse, longer term effects, including osteoporosis, lymphoedema, fatigue, weight gain, premature menopause and sexual problems. These may disrupt everyday life, social and intimate relationships and reduce life quality in the longer term. Moreover, women have reported difficulties obtaining health insurance and experienced subtle workplace discrimination.44 Many individuals may therefore require ongoing, and sometimes long-term supportive and preventative health care.
As new models of cancer service organisation and delivery emerge, practice nurses may find they are increasingly called on to support breast cancer survivors, by:
- Understanding breast cancer survivors' actual and potential needs
- Promoting and supporting health, well-being and active lifestyles to prevent or minimise the late effects of treatments, e.g. osteoporosis and weight gain
- Monitoring for and promoting the early detection of recurrence and/or late and long term effects of cancer treatments, e.g. lymphoedema
- Providing relevant, up-to-date information about local support networks
- Supporting self-management strategies, e.g. exercise regimes and relaxation.29
Indeed, while recognising the vital and invaluable role of breast cancer specialist nurses, primary care practitioners, particularly nurses and GPs, are ideally placed to work alongside the specialists to support breast cancer survivors especially (but not exclusively) in the longer term. This approach is endorsed by the National Cancer Survivorship Initiative.45
CONCLUSION
The increasing incidence and prevalence of primary breast cancer, coupled with the policy drive to treat people closer to their homes, ever improving outcomes and changing expectations will bring about new challenges for primary care practitioners. Practice nurses may find that they are increasingly called on to support women with breast cancer and their families, during and after treatments. This could be stimulating, rewarding work but breast cancer care is a dynamic field and it is incumbent upon practice nurses to be aware of key specialist contacts, existing and new treatments, their immediate and long term effects, and the needs of patients.
SELF-ASSESSMENT QUESTIONS
1. Why are more women now surviving primary breast cancer in the United Kingdom?
2. Why is breast self examination no longer recommended?
3. From what age are women in the United Kingdom invited for mammography?
4. Why, at this time, is an independent review of the research evidence for mammography screening taking place in the United Kingdom?
5. What are the three components of 'triple assessment'?
6. List the possible adverse effects of axillary lymph node dissection or clearance?
7. Why might chemotherapy include drugs from the anthracycline group?
8. What are the main side effects of aromatase inhibitors?
9. What is the epidermal growth factor found in some breast cancers called?
10. List the ways in which primary care professionals might contribute to the ongoing care and support of cancer survivors and their families.
SELF-ASSESSMENT ANSWERS
1. Advances in scientific knowledge, early detection and treatment modalities.
2. Research findings suggest increased harm in terms of greater numbers of benign lesions identified and biopsies performed.
3. 50
4. In light of research findings there is considerable debate about the benefits and harms of mammography screening.
5. Clinical assessment by breast specialist, radiology in the shape of mammography or ultrasound, cytology by fine needle aspiration or core biopsy.
6. Risk of upper limb lymphoedema, pain, decreased range of limb movement, impact on quality of life.
7. Studies have indicated that regimen's containing anthracyclines reduce recurrence and increase five year survival.
8. Musculoskeletal pain and osteoporosis.
9. HER-2
10. Provision of information, support self-management, education regarding healthy lifestyles, coordination of care, excellent communication.
"ƒ
REFERENCES
1. World Health Organization. Fact Sheets: Cancer 2012; http://www.who.int/mediacentre/factsheets/fs297/en/
2. Cancer Research UK. Breast Cancer — UK incidence Statistics 2012 CRUK.
www. http://info.cancerresearchuk.org/cancerstats/types/breast/incidence/
3. Parkin DM. Is the recent fall in incidence of post-menopausal breast cancer in UK related to changes in use of hormone replacement therapy? Eur J Cancer Care 2009; 45: 1649- 1653
4. Roberts H. Reduced use of hormones and the drop in breast cancer. BMJ 2009; 338:b2116 doi: 10.1136/bmj.b2116
5. Sharpe KH, McClements P et al. Reduced risk of oestrogen receptor positive breast cancer among peri- and post-menopausal women in Scotland following a striking decrease in use of hormone replacement therapy. Eur J Cancer 2010; 46: 937-943
6. Pelucchi C, Levi F, La Vecchia C. The rise and fall in menopausal hormone therapy and breast cancer incidence. Breast 2010; 198-201
7. Hery C, Ferlay J et al. Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann Oncol 2008; 19: 1009-1018
8. Bosetti C, Bertuccio P et al. The decline in breast cancer mortality in Europe: An update (to 2009). Breast 2012; 77-82
9. Office for National Statistics. Cancer survival rates in England for patients' diagnosed between 2001-2006 followed up 2007. 2009 ONS
10. Schopper D, de Wolf C. How effective are breast cancer screening programmes by mammography? Review of the current evidence. Eur J Cancer 2009; 1916-1923
11. Zabora JR, Morrison C et al. Recruitment of underserved women for breast cancer detection programmes. Cancer Pract 1997; 5(5): 297—303
12. Blamey RW, Wilson ARM, Patnick J. ABC of breast diseases: screening for breast cancer. BMJ 2000; 321: 689—693
13. Barnett GC, Shah M. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol (2008); 26: 3310- 3316
14. Cancer Research UK. Breast Cancer Risk Factors. CRUK 2012 http://info.cancerresearchuk.org/cancerstats/types/breast/riskfactors/breast-cancer-risk-factors#Exogenous
15. Blanks RG, Moss SM et al. Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990—8: comparison of observed with predicted mortality. BMJ 2000; 321: 665—669
16. Watts T, Merrell J et al. Promoting breast health: information needs of minority ethnic women in the United Kingdom. J Adv Nurs 2004; 47: 526-535
17. McCready T, Littlewood D, Jenkinson J. Breast self-
examination and breast awareness: a literature review. J Clin
Nurs 2005; 14: 570-578
18. Kosters JP, Gotzsche PC. Regular Self-examination or Clinical Examination for Early Detection of Breast Cancer. Cochrane Database of Systematic Reviews 2003; 2 http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003373/pdf/standard
19. Thornton H, Pillarisetti RR. 'Breast awareness' and 'breast self-examination' are not the same. What do these terms mean? Why are they confused? What can we do? Eur J Cancer 2008; 44: 2118-2121
20. Glasziou P, Houssami N. The evidence base for breast cancer screening. Prev Med 2011; 53: 100-102
21. Montgomery M, McCrone S. Psychological distress associated with the diagnostic phase for suspected breast cancer: systematic review. J Adv Nurs 2008; 66 (11): 2372-2390
22. Liau MN, Chen MF et al. Health care and support needs of women with suspected breast cancer. J Adv Nurs 2007; 60 (3): 289-298
23. Poole K. The emergence of the 'waiting game': a critical examination of the psychosocial issues in diagnosing breast cancer. J Adv Nurs 1997; 25: 273-281
24. Gilbert JE, Green E et al. Nurses as patient navigators in cancer diagnosis: review, consultation and model design. Eur J Cancer Care 2011; 20: 228-236
25. Poole K., Lyne P. The 'cues' to diagnosis: describing the monitoring activities of women undergoing diagnostic investigations for breast disease. J Adv Nurs 2000; 31 (4): 752-758
26. NICE. Improving Outcomes in Breast Cancer. 2002. National Institute for Health and Clinical Excellence, London.
27. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer 2008; 112:2577- 2592
28. Watts T. Caring for Cancer Survivors. Pract Nurse 2011; 41 (12): 40-45
29. NICE. Early and localised primary breast cancer: diagnosis and treatment. 2009; National Institute for Health and Clinical Excellence, London.
30. McCann L, Illingworth N et al. Transitional experiences of women with breast cancer within the first year following diagnosis. J Clin Nurs 2010; 19 (13-14) 1969-1976
31. Simone NL, Dan T et al. Twenty five year results of the National Cancer Institute randomized breast cancer conservation trial. Breast Cancer Res Treat 2012; 132; 197-203
32. Sakorafas GH, Sarioias M. Breast cancer surgery: an historical narrative. Part III. From the sunset of the 19th to the dawn of the 21st century. Eur J Cancer Care 2010; 19: 145-166
33. Avril A, Le Bouedec G et al. Phase III randomized equivalence trial of early breast cancer treatments with or without axillary clearance in post-menopausal patients results after 5 years of follow-up Eur J Surg Oncol 2011; 37: 563-570
34. Thomas-Maclean RL, Hack T et al. Arm morbidity and disability after breast cancer: new directions for care. Onc Nurs Forum 2008; 35: 10: 65-71
35. Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087-2106
36. Early Breast Cancer Trialists Collaborative Group. Effect of radiotherapy after breast conserving surgery on 10 year recurrence and 15 year breast cancer death: meta analysis of individual patient data for 10, 801 women in 17 randomised trials. Lancet 2011: 378; 1707-1716
37. Schnur JB, Ouellette SC et al. A qualitative analysis of acute skin toxicity among breast cancer radiotherapy patients. Psychooncology 2011; 20 (3): 260-268
38. Sjovall K, Strombeck G et al. Adjuvant radiotherapy of women with breast cancer — Information, support and side-effects. Eur J Oncol Nurs 2010; 14: 147—153
39. Halkett G., Kritjanson LJ, Lobb EA 'If we get too close to your bones they'll go brittle': women's initial fears about radiotherapy for early breast cancer. Psychooncology 2008; 17:877- 884
40. Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. 2005 Lancet; 365: 1687—1717
41. Early Breast Cancer Trialists Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378(9793):771-784
42. Boehm DU, Lebrecht A et al. Quality of life and adjuvant tamoxifen treatment in breast cancer patients. Eur J Cancer Care 2009; 18: 500—506
43. Dixon JM, Wilson V et al. HER2 testing in patients with breast cancer. BMJ 2012; 344 doi: 10.1136/bmj.e3958
44. Bloom JR, Stewart SL et al. Quality of life of younger breast cancer survivors: persistence of problems and sense of well-being. Psychooncology 2012; 21: 655-665
45. NSCI. The National Cancer Survivorship Initiative Vision. NSCI. 2010. www.nsci.org.uk
46. Wu HS, Dodd M, Cho MH. Patterns of fatigue and effect of exercise in patients receiving chemotherapy for breast cancer. Onc Nurs Forum 2008; 35: E90-E99.
47. Katsimbri P, Bamias A, Palidis N, Prevention of chemotherapy induced alopecia using an effective scalp cooling system. Eur J Cancer 2000; 36: 766—71
48. Fenlon DR, Corner JL, Haviland JS. A randomized controlled trial of relaxation training to reduce hot flashes in women with primary breast cancer. J Pain Sympt Man 2008; 35:397-405
Related articles
View all Articles